> 99.99%
EtO Destruction Removal Efficiency proven
$15M
in upgrades re-directed to expanding capacity
Guesswork Isn’t a Strategy for Compliance
In 2023, a leading medical device sterilization facility found itself on shaky ground. Emissions compliance was managed through a patchwork of manual record-keeping and periodic testing, with limited real-time insight using their existing monitoring systems. The facility’s legacy optically enhanced FTIR CEMS provided data, but not certainty. In fact, these older systems consistently over-reported actual ethylene oxide (EtO) emissions by an order of magnitude, triggering false concerns that eroded trust from sterilization managers, EHS personnel, regulators and the local community. Every anomalous reading became a fire drill – was it real or interference? Lacking confidence in the emissions data, the team lived in a state of perpetual operational anxiety. Compliance felt less like a science and more like guesswork, leaving stakeholders uneasy and employees skeptical. The company’s core mission – sterilizing life-saving medical devices – was at risk of being overshadowed by data doubts and compliance uncertainty.
The EtO Sterilization Challenge: High Stakes and Higher Costs
This operational uncertainty came to a head as new EPA regulations tightened limits of EtO emissions and both public and employee concern increased. In 2024, the EPA strengthened its rules, mandating up to 99.99% destruction of EtO emissions from large sterilization facilities. Public scrutiny was intensifying too; communities around sterilization plants, having heard reports of excessive emissions demanded transparency and accountability. Facing the prospect of penalties or even shutdowns, the sterilization company prepared to act.

But without trusted emissions data, the only safe bet seemed to be a costly one. Management earmarked $15 million for additional abatement hardware–essentially, over-engineering the emissions control systems–to create a buffer against the current processes' Destruction Removal Efficiency (DRE). However, that plan was based on unreliable monitoring data. This was a massive investment in equipment that might not even be needed, essentially an insurance policy against data uncertainty. It wasn’t an isolated case; after the Commercial Sterilizer NESHAP was released, industry analysts warned that compliance costs for the new rules would far exceed initial estimates, and they were right, as sterilizers scrambled to add extra emissions controls. The company was prepared to pour eight figures into new oxidizers and dry scrubbers, not because their existing system couldn’t destroy the gas, but because they couldn’t prove it. In the absence of a trusted monitoring solution, the company was prepared to solve a data problem with costly additional abatement.
"The company was prepared to pour eight figures into new oxidizers and dry scrubbers, not because their existing abatement system couldn’t destroy the gas, but because they couldn’t prove it."
Data-Driven Strategy: Finding a Single Source of Truth
Before finalizing a $15 million investment in new abatement hardware, the sterilization facility’s leadership noted a recurring discrepancy: legacy monitoring consistently showed elevated emissions—even while the abatement supplier asserted that they were operating at only half capacity. This inconsistency suggested that the real issue might be measurement reliability, not actual abatement performance. In response, the company sought a solution that would resolve the data gap at its core rather than simply adding more equipment.
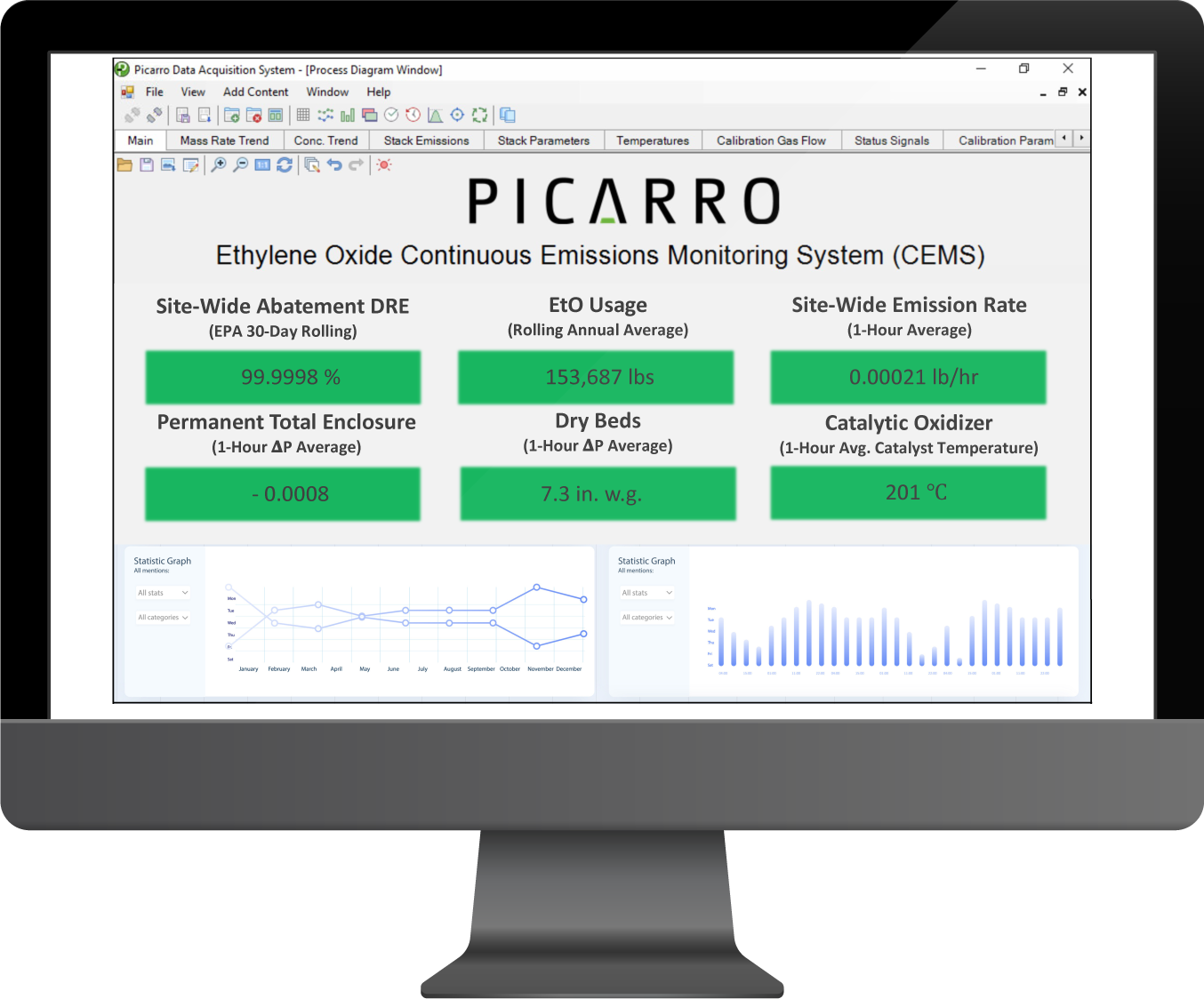
That realization led them to Picarro, whose next-generation monitoring systems—built on Cavity Ring-Down Spectroscopy (CRDS)—had a proven track record of delivering repeatable sub-ppb EtO measurements without interference. Leveraging Picarro’s turn-key solution, the site had a working implementation of CEMS within 1 week, reporting near 100% DRE with existing abatement, and passing daily calibrations at 10 ppb consistently. Picarro’s CEMS not only met but exceeded EPA Performance Specification 19 (PS 19) for NESHAP compliance. Beyond providing high data integrity, Picarro’s integrated approach came with a built-in compliance logic that automatically tracked abatement temperatures, flow rates, EtO throughput, and other critical process parameters. This solution automated all compliance calculations and provided real-time DRE complete with alert-management automation—thereby eliminating the manual, deterministic workload of verifying abatement efficiency and regulatory compliance.
Equally significant, Picarro offered an end-to-end service model, handling every aspect of the facility’s emissions management program—from certification testing and maintenance, to regulatory reporting—thus removing the burden of fragmented services and compliance cost management. As a result, the sterilization company gained one source of truth for real-time process efficiency data, saved millions on unnecessary abatement upgrades, reduced operational risk from public and regulatory intervention, and shifted focus back to its core mission of delivering safe, sterile medical devices.

"Leveraging Picarro’s turn key solution, the site had a working implementation of a CEMS within 1 week, reporting near 100% DRE with existing abatement."

Transformation: From Uncertainty to Unlocked Capacity
With the data-driven strategy implemented by leadership, the effect was immediate and dramatic. Trusted data poured in, and for the first time, the facility saw a clear picture of its emissions – one that told a very different story than the legacy monitoring systems. The existing LESNI catalytic oxidizer, it turned out, was performing extraordinarily well. Over the course of normal operations, Picarro’s system measured EtO levels going into and out of the oxidizer and calculated an actual DRE that stunned everyone. The oxidizer was destroying 99.99934% of EtO and yielding stack concentrations of barely 1–2 ppb – essentially zero from any practical perspective. This wasn’t just a hair better than required – it was an order of magnitude beyond the EPA’s mandate of 99.99% DRE.
The revelation carried immediate financial and strategic consequences. The $15 million earmarked for a new abatement system was swiftly taken off the table. With regulatory compliance now assured by empirical continuous monitoring results, there was simply no need to invest in redundant abatement hardware. Instead, those funds were redirected to core operations – specifically, expanding sterilization capacity. The company ordered new sterilization chambers and increased its EtO throughput, confident that it could scale production with data that they knew would reassure stakeholders both internal and external to the facility.
By the numbers, the facility’s environmental performance went from uncertain to undeniably world-class, and capital that was about to be sunk into “just-in-case” control devices was freed up for growth.
Strategic Outcomes: Compliance, Confidence, and Core Focus
Perhaps the most profound change was qualitative: uncertainty was replaced with visibility and confidence – from employees who long believed there was an emissions issue to local communities who couldn’t get a clear answer on risk. With continuous monitoring in place, staying within emissions limits became a worry-free aspect of daily operations – practically compliance on autopilot. Instead of scrambling to hire a consultant for data analysis and quarterly reporting or fearing surprise EPA inspections, the facility staff could pull up a live dashboard at any moment and show anyone exactly what was (or rather, wasn’t) coming out of their stack as they achieved near-zero EtO emissions. This level of transparency not only satisfied regulators; it actively rebuilt trust with skeptical stakeholders. Community members, who had been alarmed by past false reports, could breathe easier knowing the facility was making the right decisions. Internally, company employees and leadership alike felt a weight lifted: the data was no longer something to dread. As the EPA has noted, requirements for continuous emissions monitoring and reporting are designed to provide assurance to both agencies and communities that emissions are under control – and that burden, once seen as impossible, was fully realized here.
Freed from chasing operational risk issues associated with bad data, the company refocused on its core mission: producing safe, sterile medical devices at scale. The reallocated $15 million financed additional capacity that enabled the company to process a higher volume of products, reducing bottlenecks in the healthcare supply chain. In an era when sterilization demand is rising (and alternatives to EtO remain limited for many critical devices), this additional throughput can have a direct impact on the market.
By embracing a data-first approach, the sterilization leader transformed a compliance cost center into a growth enabler. It proved that when you eliminate operational uncertainty, you unlock the headroom to shore up a sterilization process and expand further. No longer bogged down by public concern, the leadership could plan for the future – opening new sterilization lines - knowing that their environmental controls were solid.
"Freed from chasing operational risk issues associated with bad data, the company refocused on its core mission: producing safe, sterile medical devices at scale."
Key Takeaways
Trusted Data is the Real Moat
In a tightly regulated industry, the greatest competitive advantage came from data credibility. By investing in superior monitoring, the company gained a level of insight and assurance that could not be matched with legacy CEMS providers. This trusted data became a protective moat, safeguarding the business against regulatory and public relations threats.
Single Source of Truth
Unifying compliance on one platform eliminated the chaos of fragmented systems and services. With all stakeholders – operations, environment health & safety (EHS), regulators, community – able to now quickly discern operational efficiency, there was no more debate or doubt about emissions levels. Decisions could be made on facts, not fear.
Managed Service = Peace of Mind
Partnering with Picarro manage the complexity of emissions monitoring allowed the facility to focus on what it does best. The managed service model meant experts handled all aspects of commissioning, maintenance, operation, regulatory tracking, and reporting. For the company, this translated to peace of mind: know they had one source of truth for their entire EtO emissions management program.
Data Clarity Enables Growth
Once emissions uncertainty was removed, the company unlocked significant financial and operational capacity. Capital that would have been tied up in redundant equipment was freed for expansion. Clear data showed precisely where they stood against regulatory limits, allowing them to confidently ramp up production without fear of violations.
Learn More
Read the Picarro-LESNI Case Study for details on how we proved near 100% DRE.
Talk to an Expert
We’re ready to answer your questions about compliance, monitoring systems, services, and more.

